- Your cart is empty
- Continue Shopping
📝 General Description:
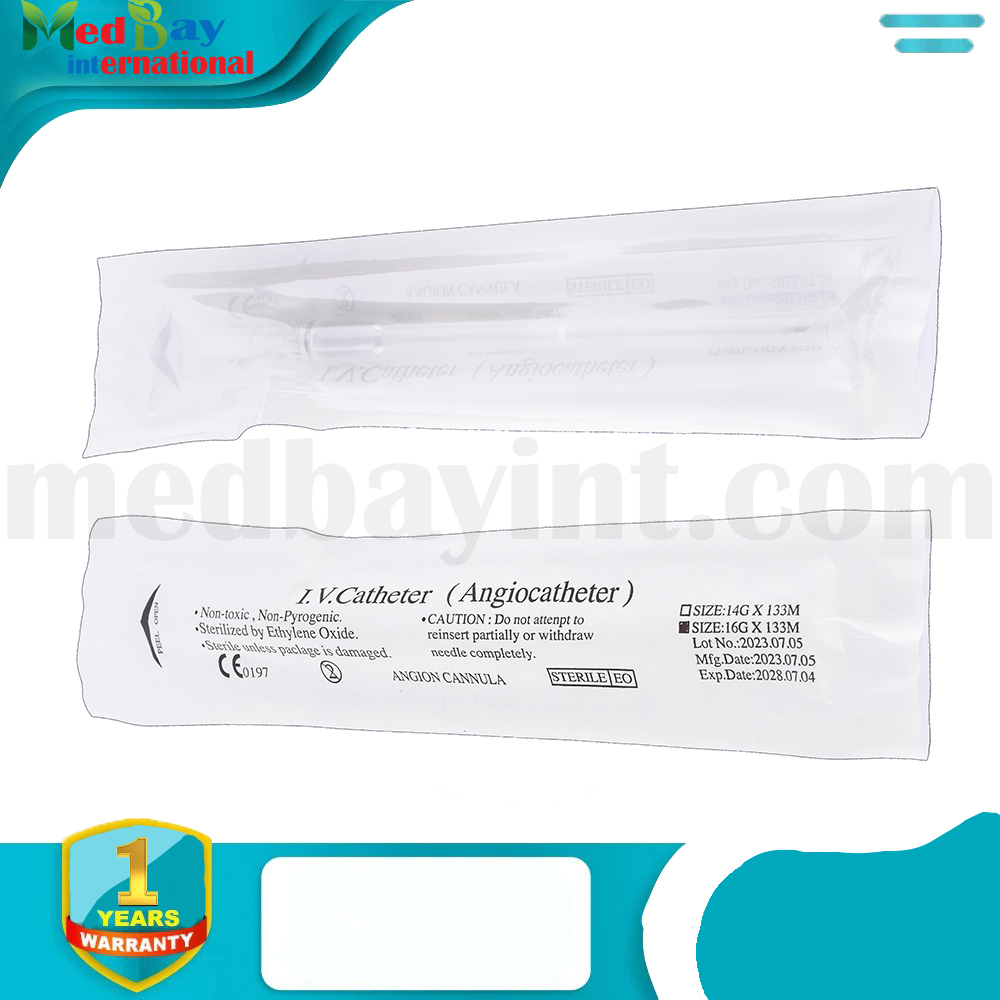
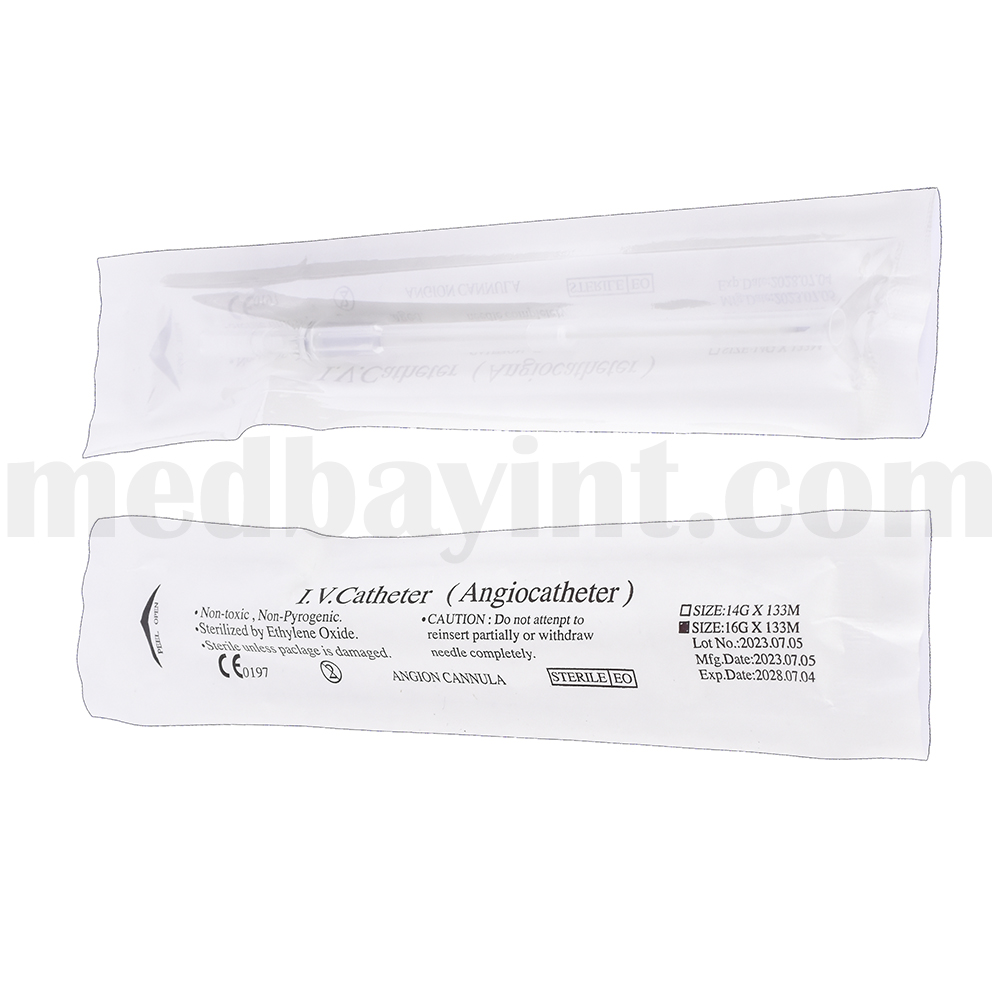
The IV Catheter (Angiocath) is a sterile, single-use peripheral venous catheter designed for secure and efficient vascular access. It facilitates the administration of fluids, medications, and blood products with minimal patient discomfort. The catheter features a thin-walled, biocompatible cannula for optimal flow rates and is designed for smooth insertion and easy withdrawal of the needle to reduce the risk of complications.
📊 Technical Specifications:
| Specification | Details |
|---|---|
| Product Name | IV Catheter (Angiocath) |
| Brand | |
| Reference Number (REF) | |
| Size | 16G × 133mm (Example: varies by product) |
| Catheter Type | Peripheral Venous Catheter |
| Usage | Single-use only |
| Material | Biocompatible polymer with stainless steel needle |
| Flow Rate | Optimized for rapid fluid administration |
| Needle Withdrawal | Smooth retraction for patient safety |
| Sterilization Method | Ethylene Oxide (EO) Sterilization |
| Application | IV fluid administration, medication delivery, emergency resuscitation |
| Storage Temperature | 10 – 30°C |
| Package Contents | Sterile, ready-to-use IV catheter |
🏥 Medical Specialties Using This Product & Applications:
- Emergency Medicine: Ensures rapid venous access for critically ill patients.
- Anesthesiology: Used for continuous administration of anesthetic drugs.
- Intensive Care Units (ICU): Facilitates long-term IV therapy for critically ill patients.
- General Medicine & Surgery: Enables safe and efficient fluid and medication administration.
📌 Main Product Category:
Vascular Access Devices
📖 How to Use:
- Remove the catheter from the sterile packaging.
- Select an appropriate vein and disinfect the insertion site.
- Insert the catheter into the vein using the beveled needle tip.
- Once blood return is observed, withdraw the needle smoothly while advancing the catheter.
- Secure the catheter and connect it to the IV line.
- Dispose of the needle safely according to medical waste regulations.
✅ Certifications & Approvals:
- FDA (U.S. Food and Drug Administration)
- CE (European Conformity)
- ISO 13485 (Quality Management for Medical Devices)
🌟 Key Features:
- Thin-walled cannula for enhanced fluid flow rates
- Smooth needle insertion reduces patient discomfort
- Biocompatible material ensures minimal risk of irritation
- Sterile and single-use to prevent infection risks
- Available in multiple sizes for different patient needs
💡 Uses & Benefits:
- Provides secure and stable venous access for medication and fluid therapy
- Reduces complications and vein trauma with smooth insertion technology
- Designed for efficient blood draws and transfusions
- Helps in emergency and intensive care situations requiring rapid venous access
⚠️ Warnings & Precautions:
- Single-use only – Do not reuse or resterilize
- For use by trained medical professionals only
- Store at the recommended temperature and away from moisture
- Dispose of used needles according to biohazard safety guidelines
Only logged in customers who have purchased this product may leave a review.






Reviews
There are no reviews yet.