- Your cart is empty
- Continue Shopping
General Description:
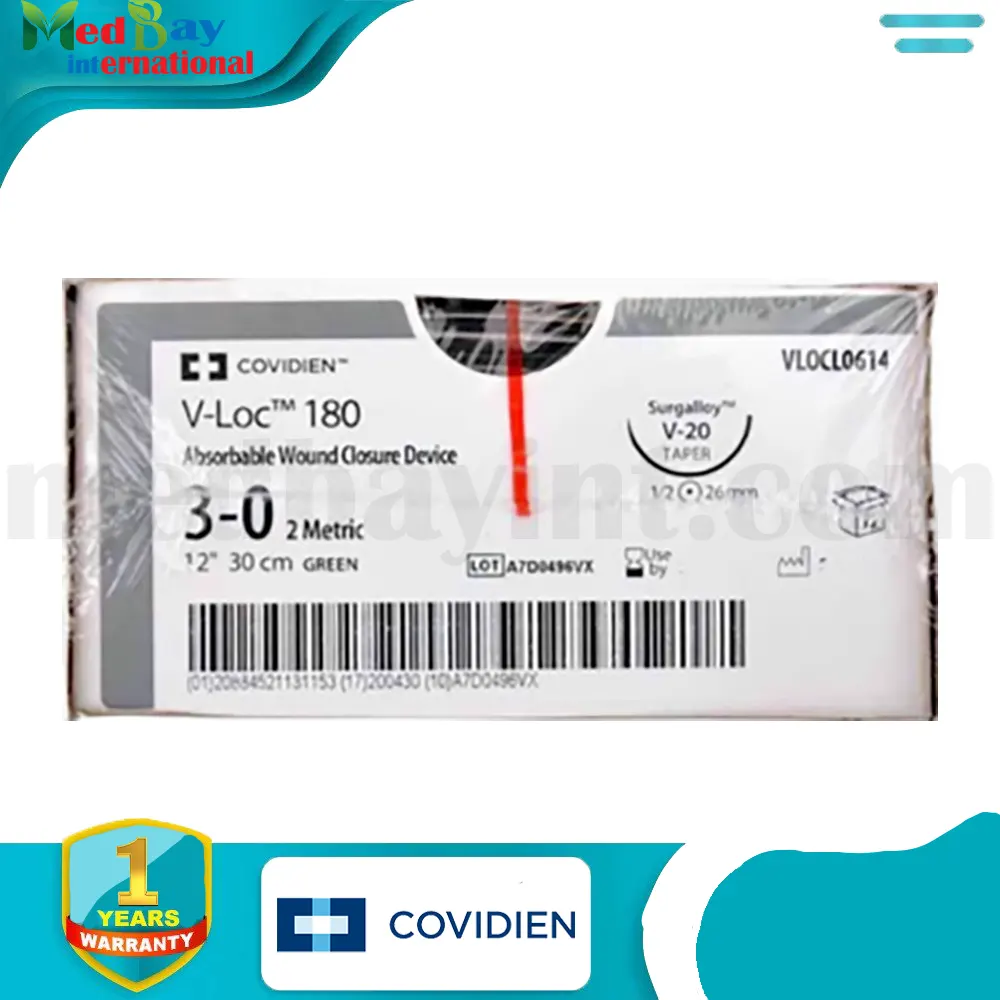
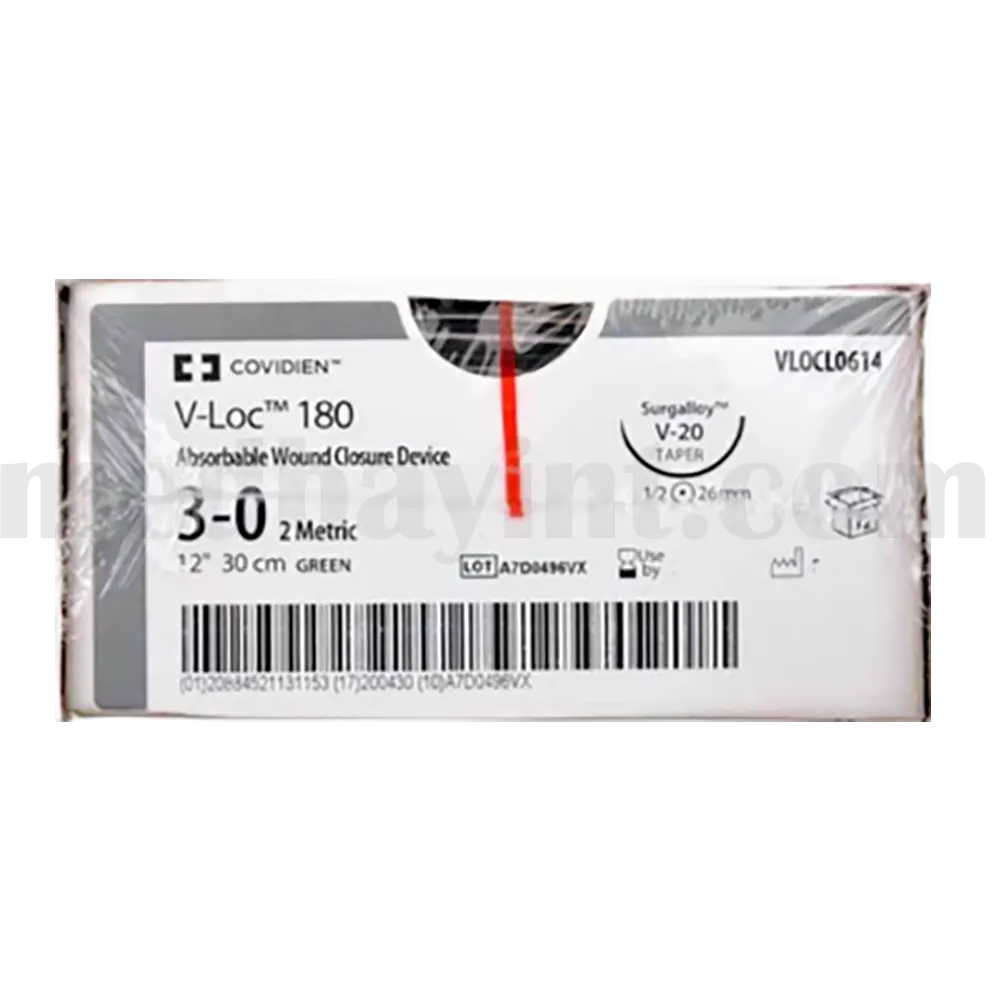
The V-Loc™ 180 Absorbable Wound Closure Device (Model VLOCL0614) is an advanced suturing solution designed for efficient, secure, and knotless wound closure. Featuring unidirectional barbed sutures, it eliminates the need for traditional knot tying, reducing operative time while ensuring tension-free tissue approximation. Made from synthetic absorbable materials, this device is ideal for various surgical applications, particularly soft tissue approximation, and is fully absorbed within 180 days.
Technical Specifications:
| Feature | Details |
|---|---|
| Brand | Medtronic |
| Product Name | V-Loc™ 180 Absorbable Wound Closure Device |
| Model Number | VLOCL0614 |
| Suture Size | Size 3-0 |
| Material | Synthetic absorbable material |
| Absorption Time | Fully absorbed within 180 days |
| Unidirectional Barbs | Provides secure, tension-free wound closure |
| Needle Type | Taper-point surgical needle |
| Usage Type | Single-use, disposable |
| Sterilization | Pre-sterilized for immediate use |
| Packaging | Individually packed in sterile wrapping |
Medical Applications:
- Wound closure in general surgeries.
- Gynecological surgeries such as cesarean sections and hysterectomies.
- Plastic and reconstructive surgeries.
- Urological surgeries.
- Soft tissue approximation in open and laparoscopic procedures.
Key Features:
- Unidirectional Barbed Suture:
- Knotless design reduces operative time and ensures secure closure.
- Absorbable Material:
- Provides sufficient tensile strength during the healing process and is fully absorbed within 180 days.
- Secure and Even Tension Distribution:
- Barbs ensure even tension along the wound, enhancing healing outcomes.
- Precision Taper-Point Needle:
- Designed to minimize tissue trauma and ensure smooth suturing.
- Ready-to-Use:
- Pre-sterilized and individually packaged for safety and convenience.
Compatibility and Usage:
- Suitable for both open and laparoscopic procedures.
- Ideal for soft tissue approximation requiring absorbable sutures.
Certifications and Approvals:
- CE Certified.
- FDA Approved.
- Compliant with international safety and quality standards.
Patient and User Benefits:
- Time-Saving: Knotless sutures accelerate wound closure, reducing surgical time.
- Minimized Tissue Trauma: Even tension distribution prevents excessive strain on tissues.
- Improved Healing Outcomes: Secure closure reduces the risk of wound reopening.
- Convenient for Patients: Absorbable material eliminates the need for suture removal.
Warnings and Precautions:
- Single-Use Only: Do not reuse or re-sterilize.
- Must be operated by trained surgical professionals.
- Store in a cool, dry place away from heat and humidity.
- Not suitable for patients with known sensitivities to absorbable materials.
Medical Specialties Using This Device:
- General Surgery.
- Obstetrics and Gynecology.
- Plastic and Reconstructive Surgery.
- Urology.
Main Product Category:
- Absorbable Wound Closure Devices.
Only logged in customers who have purchased this product may leave a review.






Reviews
There are no reviews yet.